how many valence electrons do alkali earth metals have|Alkaline earth metals: their chemical characteristics : Tagatay Reaction with halogensCa + Cl2 → CaCl2Anhydrous calcium chloride is a hygroscopic substance that is used as a desiccant. . Tingnan ang higit pa Acute myeloid leukemia (AML) is a rapidly progressing myeloid neoplasm characterized by the clonal expansion of immature myeloid-derived cells, known as blasts, in the peripheral blood and bone marrow. This expansion results in ineffective erythropoiesis and megakaryopoiesis, clinically manifesting as relatively rapid bone marrow failure .
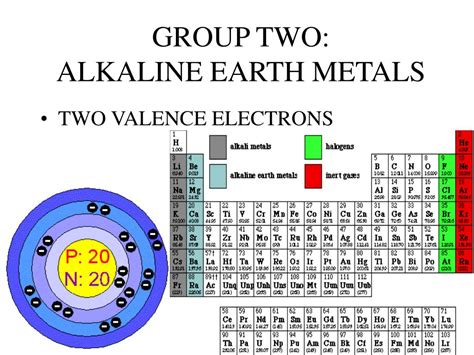
how many valence electrons do alkali earth metals have,All the alkaline earth metals have two electrons in their valence shell, so the energetically preferred state of achieving a filled electron shell is to lose two electrons to form doubly charged positive ions. Tingnan ang higit pa
The alkaline earth metals are six chemical elements in group 2 of the periodic table. They are beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra). The elements have very similar . Tingnan ang higit paChemicalAs with other groups, the members of this family show patterns in their electronic configuration, . Tingnan ang higit paMost beryllium is extracted from beryllium hydroxide. One production method is sintering, done by mixing beryl, sodium fluorosilicate, and soda at high temperatures . Tingnan ang higit paReaction with halogensCa + Cl2 → CaCl2Anhydrous calcium chloride is a hygroscopic substance that is used as a desiccant. . Tingnan ang higit pa
EtymologyThe alkaline earth metals are named after their oxides, the alkaline earths, whose old-fashioned names were beryllia, magnesia Tingnan ang higit pa
Beryllium occurs in the Earth's crust at a concentration of two to six parts per million (ppm), much of which is in soils, where it has a concentration of six ppm. Beryllium is one of . Tingnan ang higit paBeryllium is used mainly in military applications, but non-military uses exist. In electronics, beryllium is used as a p-type dopant in . Tingnan ang higit paIn contrast, the alkaline earth metals generally have little or no tendency to accept an additional electron because their ns valence orbitals are already full; an added .
Alkaline earth metals: their chemical characteristicsHow many electrons are in the outer shell of the alkaline earth elements? Are the alkaline earth elements more or less reactive than the alkali metals? Explain your answer.

In contrast, the alkaline earth metals generally have little or no tendency to accept an additional electron because their ns valence orbitals are already full; an .
The alkaline-earth (Ae) metals of group 2 of the periodic table of elements traditionally belong to the class of main-group atoms and their valence electrons occupy the ( n )s atomic orbital in the 1 S .Since all the alkaline metals have only two valence electrons, it takes little energy to make them give up those electrons to form cations (2+), resulting in high reactivity [7]. Beryllium (Be) does not react with water due to its .Alkaline earth metals have two valence electrons. They have low ionization energy, low electron affinity, and low electronegativity. They are highly reactive and often form .
As with the alkali metals of Group 1 (Ia), the atoms of the alkaline-earth metals easily lose electrons to become positive ions (cations). Most of their typical compounds are therefore ionic: salts in .
The alkaline earths have two electrons in the outer shell. They have smaller atomic radii than the alkali metals. The two valence electrons are not tightly bound to the nucleus, so the alkaline earths .
All alkaline earth metals possess two valence electrons, or electrons found in the outer shell of an atom. Due to having relatively low ionization energies for these first two electrons,. Tell which group of elements (alkali metals, alkaline earth metals, halogens, noble gases) has atoms with the specified number of valence electrons? 1 valence electron; 2 valence electron; 7 valence electrons; 8 valence electrons; Answer A. alkali metals. Answer B. alkaline earth metals. Answer C. halogens. Answer D. .The Concept of Open Valence ("Valence") The valence (or valency) of an element is a measure of its combining power with other atoms when it forms chemical compounds or molecules.The concept of valence was developed in the last half of the 19th century and was successful in explaining the molecular structure of many organic compounds.
Alkaline earth metals have 2 valence electrons. This makes them reactive, but less so than alkali metals, which have 1 valence electron. Valence electrons; Group 1 (I) (alkali metals) 1: Group 2 (II) (alkaline earth metals) 2: Groups 3-12 (transition metals) 2* Group 13 (III) (boron group) 3: . The alkaline earth metals are beryllium, magnesium, calcium, strontium, barium, and radium. Beryllium, strontium, and barium are rare, and radium is unstable and highly radioactive. . How Many Valence Electrons to Alkaline Earth Metals Have? Valence electrons refer to the number of electrons found in the outermost shell of an atom. Alkaline earth metals are characterized by .All alkali metals ( belongs to group 1 of periodic table) have 1 valence electron. Valence electron are those electron that are present in the outermost orbit of the atom. Alkali metal present in group 1 of periodic table: Hydrogen ( H ), Lithium ( Li ), Sodium ( Na ), Potassium ( K ), Rubidium ( Rb ), Cesium ( Cs ),and Francium ( Fr ). "Since the alkali metals only have one valence electron, they typically achieve this state by giving up that electron. In this process, the alkali metal is said to be oxidized, and whatever takes the electron from the alkali metal is reduced. All of the alkali metals like to give up their single valence electron," says Dr. Chip Nataro . Due to this, alkali metals with a single valence electron are more reactive than alkaline Earth metals, which have two valence electrons. Uses of alkali metals. Many things can be made out of pure sodium, including highly effective lamps that utilize sodium vapor. A vital role in the biological system is played by potassium. How many valence electrons do halogens have? . It is an Alkaline earth metal and is located in Group 2 of the periodic table. it has the symbol Ba. Caesium. Caesium (Cs) is a soft gray coloured metal that has the atomic number 55 in the periodic table. It is an Alkali Metal and is located in Group 1 of the periodic table. it has the .how many valence electrons do alkali earth metals have As with the alkali metals of Group 1 (Ia), the atoms of the alkaline-earth metals easily lose electrons to become positive ions (cations). Most of their typical compounds are therefore ionic: salts in which the metal occurs as the cation M 2+ , where M represents any Group 2 atom.
how many valence electrons do alkali earth metals have Alkaline earth metals: their chemical characteristics Calcium is the 20th element in the periodic table. It is a group 2 metal, also known as an alkaline-earth metal, and no populated d-orbital electrons. Calcium is the fifth most abundant element by mass (3.4%) in both the Earth's crust and in seawater. All living organisms (in fact, even dead ones) have and need calcium for survival. Calcium .In addition to solvated electrons, solutions of alkali metals in liquid ammonia contain the metal cation (M +), the neutral metal atom (M), metal dimers (M 2), and the metal anion (M −). The anion is formed by adding .How many valence electrons do the halogens, the alkali metals, and the alkaline earth metals each have, respectively? 2, 4, 6. 1, 5, 7. 8, 2, 3. 7, 1, 2

All alkali metals have one electron in their outer shell. The outer shell of an atom's electron cloud is called the valence shell and the electrons in.We would like to show you a description here but the site won’t allow us. Although many of these properties are similar to those of the alkali metals (Table \(\PageIndex{1}\)), certain key differences are attributable to the differences in the valence electron configurations of the two groups (ns 2 for the alkaline earth metals versus ns 1 for the alkali metals). The other alkali metals and the alkaline earth metals are recovered from their ores by similar processes. General Properties of the Alkali Metals. Various properties of the group 1 elements are summarized in Table \(\PageIndex{1}\). . is attributed to a net transfer of the valence electron of the alkali metal to the graphite layers to produce
Atoms are most stable if they have a filled valence shell of electrons. . Alkali metals – Group 1 (I) 1: Alkaline earth metals – Group 2 (II) 2: Boron group – Group 13 (III) 3: Carbon group – Group 14 (IV) 4: Nitrogen group – Group 15 (V) 5: .
how many valence electrons do alkali earth metals have|Alkaline earth metals: their chemical characteristics
PH0 · What Are the Properties of the Alkaline Earth Metals?
PH1 · The Valence Orbitals of the Alkaline
PH2 · Chapter 20.4: The Alkaline Earth Metals (Group 2)
PH3 · Alkaline earth metals: their chemical characteristics
PH4 · Alkaline earth metal
PH5 · Alkaline Earth Metals: Definition & Location in the Periodic Table
PH6 · Alkaline Earth Metals: Definition & Location in the
PH7 · Alkaline Earth Metals
PH8 · Alkaline
PH9 · 6.10: Alkaline Earth Metals
PH10 · 20.5: The Alkaline Earth Metals (Group 2)